Hello,
After many comments
and requests, I have returned to writing my monthly (possibly even more often)
science emails. This month, I will cover an interesting topic that peaked my
interest recently as it delves into the Science from between two different fields....Biology
and Physics.
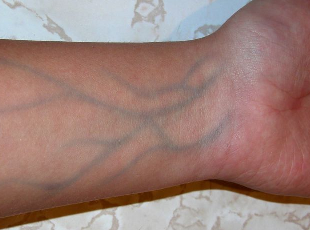
Q: "Why are my
veins blue, if my blood is red"?
A: "Your veins
are actually red, they just appear blue to the observing eye".
Wait, what? Yes,
seriously, what you see as blue is actually red. Confused? Ok, let me explain.
And no, it has very little to do with the lack of oxygen in veins returning to
the heart.
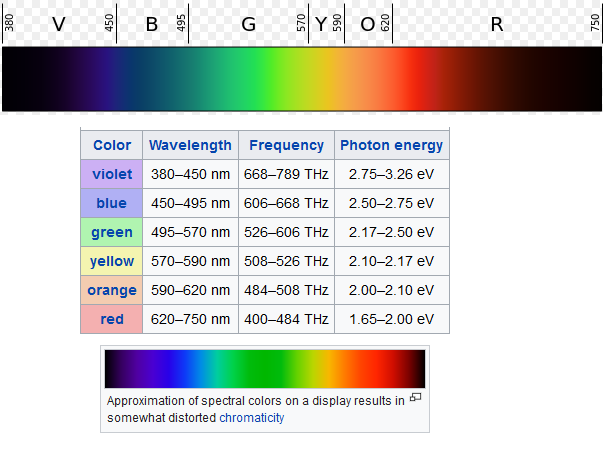
Visible light is split
into different wavelengths throughout the entire color spectrum. The color
spectrum is in itself a very small portion of the electromagnetic spectrum. For
example:
So what does that have
to do with my veins appearing as 'blue' even though they are 'red'?
Alright...now to answer that question lets delve even deeper into this rabbit
hole called Science. We need to learn a little about light absorption,
scattering, refraction and reflection. We will focus on Absorption and Reflection for
the purpose of this article to keep things simple. Each wavelength of visible
light which has a certain energy potential. Notice on the chart above how Red
has about 1.65-2.00 electron volts of energy, whereas the color Blue has
2.50-2.75 Electron volts of Energy. We call this energy potential the level of
"Excitation".
Also worth noting in
the chart for the purpose of this explanation is that the wavelength for blue
is far shorter at 450-495 nano meters as opposed to
red with 620-750 nano meters. A good analogy to
understand wavelengths is to take an incoming horizontal wave and have hit a
wall with a slit only big enough for a smaller wave.
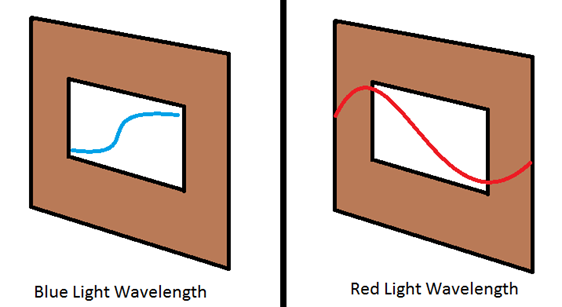
Notice how blue light
has a a shorter wavelength and can get through the
small opening, and red light being a longer wavelength will be blocked and not
make it through. In this drawing, the red light would then be observed. Of
course this is a wildly simplified analogy and I haven't even gone into the
debate of light being a wave function or a particle, but that too is a subject
for another monthly science email to come. Different materials, like water,
glass, wood, skin and even air have different chemistry which are better at
absorbing some colors better than others based on their particular light
absorption and reflection characteristics at the atomic level. Ultraviolet
radiation is, as you might have guessed, light from our Sun/Star. When this
light hits our skin on a hot summer day what happens? A large percentage of the
light is absorbed and converted into heat energy and causes your skin to get
warm.
The remaining light
that isn't absorbed is scattered or reflected. Energy is never lost or
destroyed, just converted into other forms of energy in itself. Some of these
particles even carry enough energy to be absorbed down far enough to damage DNA
in our skin and cause DNA coding errors which can lead to Skin Cancer. Which is
why it's good to always wear sunscreen which will reflect more light away from
the skin rather than absorbing it. Which is why you always feel a little bit
cooler after applying sunscreen than without, as a larger portion of the light
energy is reflected rather than absorbed and converted into heat energy.
Now, getting back the
blue vein question:
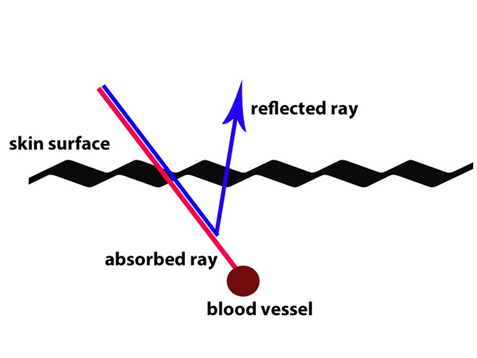
As you can see in this
diagram above. Red light wavelengths are more likely to be absorbed beyond the
skin surface and the red color will not bounce back to our eyes as much as
blue. Blue light tends to reflect much better in the biological makeup of the skin
and blood vessels. Even though the rumor about veins being blue because they
have no oxygen, which many of us have been told, is not completely true, there
is some truth to this rumor after all. Oxygenated blue does absorb more blue
light then blood without as much oxygen, which is why arteries appear more red
than blue to our eyes as they are carrying oxygen to our organs. But for the
most part, the blue light being reflected back more so than the red is much
more to do with the particular cells and their chemistry in our skin. So when
we accidentally cut ourselves, our blood is definitely red and has nothing to
do with exposure to oxygen in the least.
Now that we have
learned a little something about colored wavelength absorption, I think for the
next science email, we take this one step further and explain how astronomers
are able to detect what the atmosphere of a distant planet in a distant galaxy
over millions of light years away is made up of. The answer to this is very
intriguing and ingenious and uses the basis of what we've just learned
answering the query about blue veins. How does the question about blue veins
tell us about Exo-planet atmospheric chemistry
detection? Stay tuned to find out!!!
Thanks everyone for
the continual encouragement and feedback with my Science Emails. I hope I have
widened and educated some minds by sharing my science knowledge with others.
There is so much joy in understanding more about the world around us and how it
works.
Hope you have a great
week and remember to keep learning every day,
Tyler Waldrop